- Valence Electron Configuration Chart
- S Element Valence Electrons
- Element S Valence Electrons
- S Number Of Valence Electrons
- S Valence Electrons
- S Orbital Valence Electrons
- Sulfur S Valence Electrons
Main Difference – Valence Electrons vs Free Electrons
An atom is composed of three types of subatomic particle: electrons, protons, and neutrons. Protons and neutrons are in the nucleus of the atom. Electrons are located outside the nucleus. These electrons are in continuous movement around the nucleus at certain distances. The pathways that these electrons move in are called electron shells or orbitals. One atom can have one or more orbitals. Valence electrons are the electrons that can be found in the outermost orbital of an atom. Free electrons are not bound to atoms. These electrons can be found in lattice structures. They are in free movement inside the lattice. The main difference between valence electrons and free electrons is that the number of electrons is an elemental property whereas the number of free electrons is a lattice property.
Valence electrons are the outer electrons that are involved in bonding. Only electrons in the s and p orbitals are valance electrons, so a given atom can have between 0 and 7 valance electrons. Atoms with 0 valence electrons are called noble gases and don t like form bonds. In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus.The closest shell to the nucleus is called the '1 shell' (also called the 'K shell'), followed by the '2 shell' (or 'L shell'), then the '3 shell' (or 'M shell'), and so on farther and farther from the nucleus.The shells correspond to the principal quantum numbers (n. The electrons of an atom are typically divided into two categories: valence and core electrons. Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy levels. Valence electron definition is - a single electron or one of two or more electrons in the outer shell of an atom that is responsible for the chemical properties of the atom. Valence is an older term that describes how many other atoms an atom can bond with. Valence electrons are the number of outer shell electrons an atom has.
Key Areas Covered
1. What are Valence Electrons
– Definition, Examples, Effect on the Oxidation State
2. What are Free Electrons
– Definition, Occurrence
3. What is the Difference Between Valence Electrons and Free Electrons
– Comparison of Key Differences

Key Terms: Atom, Atomic Number, Electrons, Free Electrons, Lattice, Metal, Neutrons, Nucleus, Orbital, Protons, Valence Electrons
What are Valence Electrons
Valence electrons are electrons present in the outermost orbitals of an atom. These are the electrons that have the least attraction towards the nucleus of an atom. This is because valence electrons are located in a long distance than other electrons of that atom.
Valence electrons are responsible for chemical reactions and chemical bonding of an atom. Since the attraction between valence electrons and the nucleus of an atom is less, valence electrons can easily be removed (than the electrons in the inner orbitals). This is important in the formation of ionic compounds and covalent compounds. By losing valence electrons, atoms can form cations. Sharing valence electrons of an atom with the valence electrons of another atom forms covalent bonds.
Group in the Periodic Table | Number of Valence Electrons |
Group 1 (ex: Na, K) | 1 |
Group 2 (ex: Ca, Mg) | 2 |
Group 13 (ex: B, Al) | 3 |
Group 14 (ex: C, Si) | 4 |
Group 15 (ex: N, P) | 5 |
Group 16 (ex: O, S) | 6 |
Group 17 (ex: F, Cl) | 7 |
Group 18 (ex: He, Ne) | 8 |
For s block elements and p block elements, the valence electrons are in the outermost orbital. But for transition elements, the valence electrons can be present in inner orbitals as well. This is due to the energy difference between the suborbitals. For example, the atomic number of Manganese (Mn) is 25. The electron configuration of cobalt is [Ar] 3d54s2. The valence electrons of cobalt should be in the 4s orbital. But there are 7 valence electrons in Mn. The electrons in the 3d orbital are also considered as valence electrons because the 3d orbital is located outside the 4s orbital (the energy of 3d is higher than the 4s orbital).
The oxidation state of an atom depends on the valence electrons of that atom. Some atoms remove valence electrons in order to get stabilized. Then the oxidation state of that atom increases. Some atoms gain more electrons in the outermost orbital. Then the number of valence electrons of that atom increases. It decreases the oxidation state of the atom.
What are Free Electrons
Free electrons are electrons that are not attached to an atom. Free electrons cannot be found everywhere. This is because a lone electron is very reactive and can react with anything. But in crystal structures and metals, free electrons can be found.
Free electrons are the delocalized electrons from the lattice. In crystal structures, some electrons do not remain in their place due to crystal defects. They become free electrons that can move anywhere inside the lattice. These electrons are responsible for the conduction of heat and electricity.
In metals, there are free electrons between metal ions. It is a lattice of metal ions in a sea of free electrons. These free electrons can conduct heat and electricity through the metal. These free electrons can conduct an electrical current via the metal.
Difference Between Valence Electrons and Free Electrons

Definition
Valence Electron Configuration Chart
Valence Electrons: Valence electrons are the electrons present in the outermost orbitals of an atom.
Free Electrons:Free electrons are electrons that are not attached to an atom.
Attraction to the Nucleus
Valence Electrons:Valence electrons have less attraction towards the nucleus of an atom.
S Element Valence Electrons
Free Electrons: Free electrons have no attraction towards the nucleus of an atom.
Chemical Bonding
Valence Electrons:Valence electrons are responsible for the chemical bonding of an atom.
Free Electrons: Free electrons are not involved in chemical bonding.
Conduction of Heat and Electricity
Valence Electrons:Valence electrons cannot conduct heat and electricity.
Free Electrons: Free electrons are responsible for the conduction of heat and electricity.
Nature
Valence Electrons:The number of valence electrons is an elemental property.
Free Electrons:The number of free electrons is a lattice property.
Conclusion
Valence electrons are electrons that are loosely bound to an atom. Free electrons are completely unbound to any atom. Valence electrons are responsible for the chemical reactions and chemical bonding of atoms. Free electrons take part in heat and electricity conduction of a lattice structure. There are many differences between valence electrons and free electrons. The main difference is that the number of electrons is an elemental property whereas the number of free electrons is a lattice property.
References:
1. “Valence electron.” Wikipedia, Wikimedia Foundation, 29 Oct. 2017, Available here.
2. “The Free Electron.” NDT Resource center, Available here.
Image Courtesy:
1. “Carbon diagonal rule” By CK-12 Foundation (raster), Adrignola (vector) – File:High School Chemistry.pdf, page 317 (CC BY-SA 3.0) via Commons Wikimedia
Element S Valence Electrons
Valence electrons
Valenceelectrons are the electrons found in outermost shell surrounding an atomicnucleus. Valence electrons are important because they provide a deep insightinto the chemical properties of an element: either electronegative orelectropositive in nature, or indicate the bond order of a chemicalcompound–the number of bonds that can be formed between two atoms. Due to theexchange of electrons present in the final shell, covalent bonds are formed,the number indicates how many bonds are allowed to form.
The most palpable method would be to refer to the atomic configuration of an element and simply count in the outermost shell the electrons. This would be an extremely tedious chore, however, as we may have to rummage through textbooks to search for configurations that we are not familiar with.
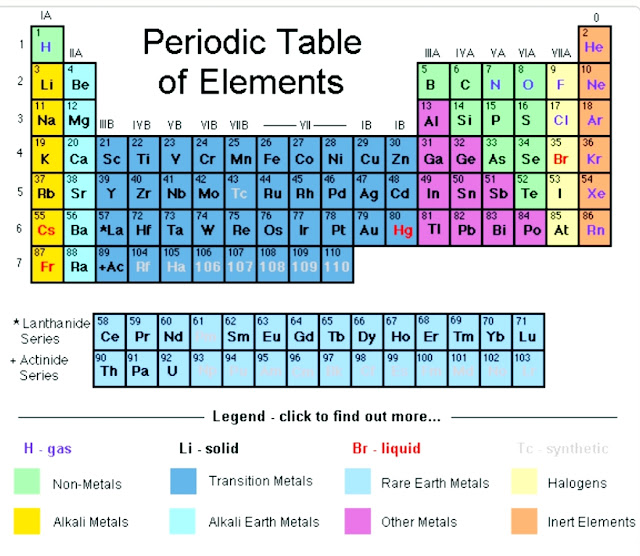
There’s noneed to worry, though, because there’s a much simpler way to find that covetednumber. This is a more generalized approach that requires only one small,shiny, rectangular sheet of paper— the periodic table — to be summoned. To findan element’s number of valence electrons, we only need to refer to the periodictable and look for the position of the element in it.
Valence electrons Periodic Table
The periodic table is a simple collection of all the elements that we have so far found. In ascending order of their atomic numbers, or the number of protons and electrons they contain, the elements are grouped from left to right.
The elements are divided into four categories: main group elements, transition elements, lanthanides and actinides. The latter two are also referred to as elements of internal transition elements. The table contains a total of 18 columns, formally referred to as groups as well as rows, formally referred to as periods. In the sub table above there are 7 rows and 2 rows that distinguish the rarer elements below.
Although thenumber of shells increasing, the number of valence electrons stays the same asthey move down a group. Although valence electrons increase by one over aperiod of time, the number of shells remains the same. The period number inwhich an item can be found (row number, to remind you) shows the number ofshells surrounding its nucleus.
Characteristics of Valence Electron
Electrons are involved in the chemical bonding of the atomand its reactions. It is said in an atom that it occupies orbitals. The numberof an atom’s valence electrons can be derived from the periodic table as it isequal to the atom’s group number. Atoms are most stable when they have a filledvalence shell of electrons. Atoms transfer or share electrons so that a filledshell of electrons can be achieved.
Some of valence electron’s key features are;
- Thevalence electron only occurs in the outermost electron shell for the main groupcomponents.
- Theremay be a valence electron in a transition metal’s inner shell.
- Typically,an atom consisting of a closed shell of valence electrons is chemically inert.
- Avalence electron in the form of a photon can either absorb or release energy.
- Theelectrons of Valence also determine an element’s electrical conductivity.Depending on the nature of the elements, a metal, nonmetal, or metalloid can beused.
S Number Of Valence Electrons
Oxygen valance electron
Oxygen is in Group VI, so the oxygen ATOM contains sixvalence electrons.
Z = 8. For oxygen If the oxygen nucleus has eight protons,the atom must also have eight negative charges, i.e. eight electrons.
Two of these electrons are the inner core and are notintended to be involved in bonding. The remaining 6 are valence electrons thatare involved in bonding and structure influences. Normally, two of theseelectrons combine to form covalent bonds with the electrons of donor atoms (cf.hydrogen). The remaining 4 valence electrons live in lone pairs that are stereochemically active and affect structure.
Carbon Valence electrons
There are 4 valence electrons in carbon atoms.
S Valence Electrons
Next to the “A” number is the number of valenceelectrons in the atom of an element in that group.
S Orbital Valence Electrons
Carbon has 4 valence electrons in Group 4A.
Also Read – Coulomb’s law – Equation, Limitations,Vector Form
Also Read – Ionization energy
Sulfur S Valence Electrons
Also Read – Electron configuration
Also Read – What Is Electric Charge
